I sincerely apologize for the delay in writing this post. Exams arose and writing for this blog had to be pushed back because, contrary to what you may believe, I have more important matters to deal with! Good news though, I'm back! And I shall keep my promise: let's discuss water - the elixir of life.

Wasser
Many believe that life originated in an environment that was naturally aquaeous. Of course, that means there must have been some water casually lying around somewhere. Despite the fact that most living things are solid objects, they are actually composed of something between 65 and 90% water. The average human body cell is made up of 80% water.
The molecule
When two hydrogen atoms form covalent bonds with an oxygen atom, a molecule of water is formed! A water molecule is actually relatively unreactive but when in the presence of other water molecules, presents some amazing, exclusive features. Water molecules have a bent, shape and are in a trigonal planar. If you consider the lone pairs of electrons on the oxygen atom, the arrangement of all the bonds are actually something close to a tetrahedral. However, the lone pairs each ''add'' an extra 2.5 degrees to the angle size between the lone pair and the hydrogen-oxygen bond because they're closer to the oxygen nucleus. The way I see it, the lone pair has a greater ''weight'' to it and its shorter distance to the oxygen nucleus makes it more influential in relation to the whole molecular shape. In a perfect tetrahedral-shaped molecule (like methane, CH4), the bond angles are 109.5 degrees each. Therefore, in water (which has two lone pairs, remember), the bond angles between the oxygen central atom and each hydrogen atom must be around 104.5 or 105 degrees.
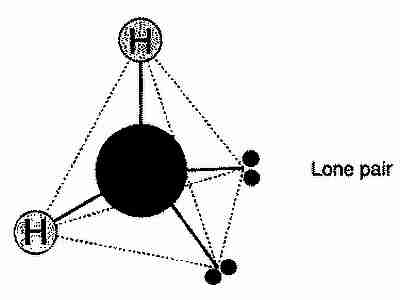
Here's the nitty-gritty maths:
Lone pair = 2.5 degrees
2 x Lone pairs = 2 x 2.5 = 5 degrees
109.5 - 5 = 104.5 degrees or 105 degrees (3sf)
Hydrogen bonding in water
Water molecules are neutral electrically. However, due to the electronegativity of the oxygen and the resulting polarity of the molecule, the oxygen atom has a net negative charge and the hydrogen atom has a net positive charge.
All molecules form vdw forces with each other. But water molecules form hydrogen bonds with each other, the strongest type of intermolecular force I know.

Some substances that dissolve in water are those that form hydrogen bonds which are stronger than the hydrogen bonds that already exist between the water molecules. Other substances, like tablesalt (NaCl), dissolve as a result of having water molecules surround them in a peculiar way (but a way that actually, when you think about it, makes a lot of sense). Here's a beautiful pic:

Oh, and the reason there is a ''2'' in front of the oxygen's delta negative charge is because there are two lone pairs. One lone pair would theoretically cause a single delta negative charge (1).
That's it for now, next time I wanna discuss molecules with a carbonyl group. After hearing a lecture on biochemistry earlier this month, I learnt that carbonyl groups feature very heavily in biochemistry as a general subject. It makes sense to find out a little more about it, right? Until then folks.
Exocytosis