Lipids are organic molecules. That means they have carbon in them. They also contain oxygen and hydrogen. However, compared to carbohydrates (which also constitutes these three elements), the proportion of oxygen compared to the other elements is considerably less in a lipid.
One thing that characterises lipids from other molecules is the fact that it is insoluble in water. This is why lipids are used in the manufacturing of cell-surface membranes, but we'll get there later.
They are insoluble and so, we can call them hydrophobic. They hate water. However, they are soluble in ethanol (an alcohol) and ether (an...ether). These substances are organic solvents.
Three classifications of lipids exist:
1. Simple lipids e.g. vegetable oils
2. Phospholipids
3. Steroids (what?!)
Why do we need lipids?
Well we need fats for insulation. We are kept warm by layers of fat and this can especially be seen in seals which have thick blubber to deal with the harsh cold winters of the polar regions of Earth.
Plants use lipids to form their waxy cuticles. Wax is a simple lipid. Therefore, plants need lipids for waterproofing (which is what the waxy cuticle does).
Lipids are essential in the formation of cell-surface membranes. This applies to almost all organisms. I think an exception would have to be viruses - they haven't got any cell-surface membranes. I'm not even sure if I would even accept them as living organisms... but we'll look into that another time!
Fats are energy stores. In the short term, we are fuelled by carbohydrates, but the fats are the big ones. They can be burned off very slowly and many animals use it for hibernation.
There are tonnes of other uses too. Trust me.
The test for a lipid
This is really simple. Laughably so. If you add water to a solution of lipid that has been dissolved in ethanol then a cloudy white emulsion is formed as a result.
Fats and oils
Many people often mistake them as being macromolecules. Actually, due to their hydrophobic properties, they tend to cohere together to form massive globular droplets (when I say massive, I mean massive relative to other molecules - for us, they're literally just droplets).
Fats are solids at room temperature.
Oils are liquids at room temperature.
Okay, take a fatty acid. Take an alcohol (say, I don't know, glycerol!). Allow them to react and give off water in a condensation reaction. Esters will be formed and they are the fats and oils. Ester bonds are the (-COO-) linkages between the fatty acid and the glycerol. Of course, you have to take away a molecule of H20 away first.
 |
| Triglycerides have been formed from the condensation of three glycerol molecules. The ''ide'' comes from the ester bond formation. The ester bonds around the oxygen atom that joins the fatty acid to the glycerol. |
Phospholipids
If one of the fatty acid groups in a triglyceride molecule was replaced by a phosphate group and a chloine group was attached to a charged oxygen ion on the phosphate group, then you form what is shown below.
This exists in our cell-surface membranes and a whole stack of them forms the phosopholipid bilayer.
 |
| About the cholesterol: it just adds mechanical support amongst the phospholipids. |
They are made up of complex carbon rings that exist in both plants and animals. Chloesterol is a steroid that gives rise to bile salts and the sex hormones. It, inevitably, exists in mammals. There is another steroid that exists in our skin and is converted to vitamin D through the introduction of ultraviolet rays from the Sun. Steroids have a hydrophillic part and a hydrophobic part. The hydrophilia, in this instance, is caused by the OH group. The lone pairs on the oxygen atom in the hydroxyl group are attracted to the slightly (delta) positive charge on the hydrogen atom of a bent water molecule. The hydrophobic part of the molecule is composed largely of the carbon rings.
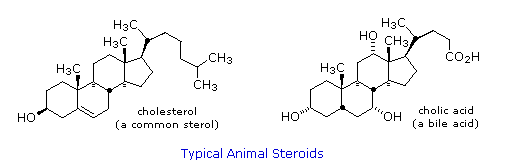 |
| Can you spot the hydroxyl groups on both steroids? |
Exocytosis